Our Solution
CytaCoat have developed a unique anti-fouling, anti-biofilm hydrogel – a non-toxic, no-kill solution that prevents biofilm (EPS – extracellular polymeric substances). The coating contains only biocompatible and organic components.
The coating is covalently bonded to the medical device and is permanent, maintaining its properties over the entire use of the product in the patient. It prevents biofilm formation to maintain a continual anti-fouling environment.

Evidence of effectiveness – Biofilm Assay
Device related infections often start with primary adhesion and colonization of microorganisms on to the device surface. After a while the microorganisms will form a biofilm (EPS) in which the microorganisms are protected against antibiotics and the immune system. The formation of a biofilm (EPS) often leads to infection and/or encrustation and the device need to be removed. CytaCoat has therefore, in collaboration with external, independent experts developed an in vitro biofilm assay.
Biofilm Assay:
In this assay Incubation of device samples (e.g. catheters, coated and non-coated) with bacteria is undertaken in a suitable media [e.g. artificial urine medium (AUM)] and biofilm (EPS) is evaluated by microscopy and immunostaining.
CytaCoat LIP Foley catheters, for example, have been tested during incubation for up to 28 days for the most common urinary tract bacteria. Coated catheters have been compared to uncoated ones and this assay has been performed for multiple strains, for example, against the most common uropathogens which form biofilm on urinary catheters and cause urinary tract infections:
- Escherichia coli
- Klebsiella pneumoniae
- Pseudomonas aeruginosa
- Proteus mirabilis
- Staphylococcus epidermidis
- Enterococcus faecalis
- Enterococcus faecium
- Staphylococcus aureus
- Fungus: Candida Albicans
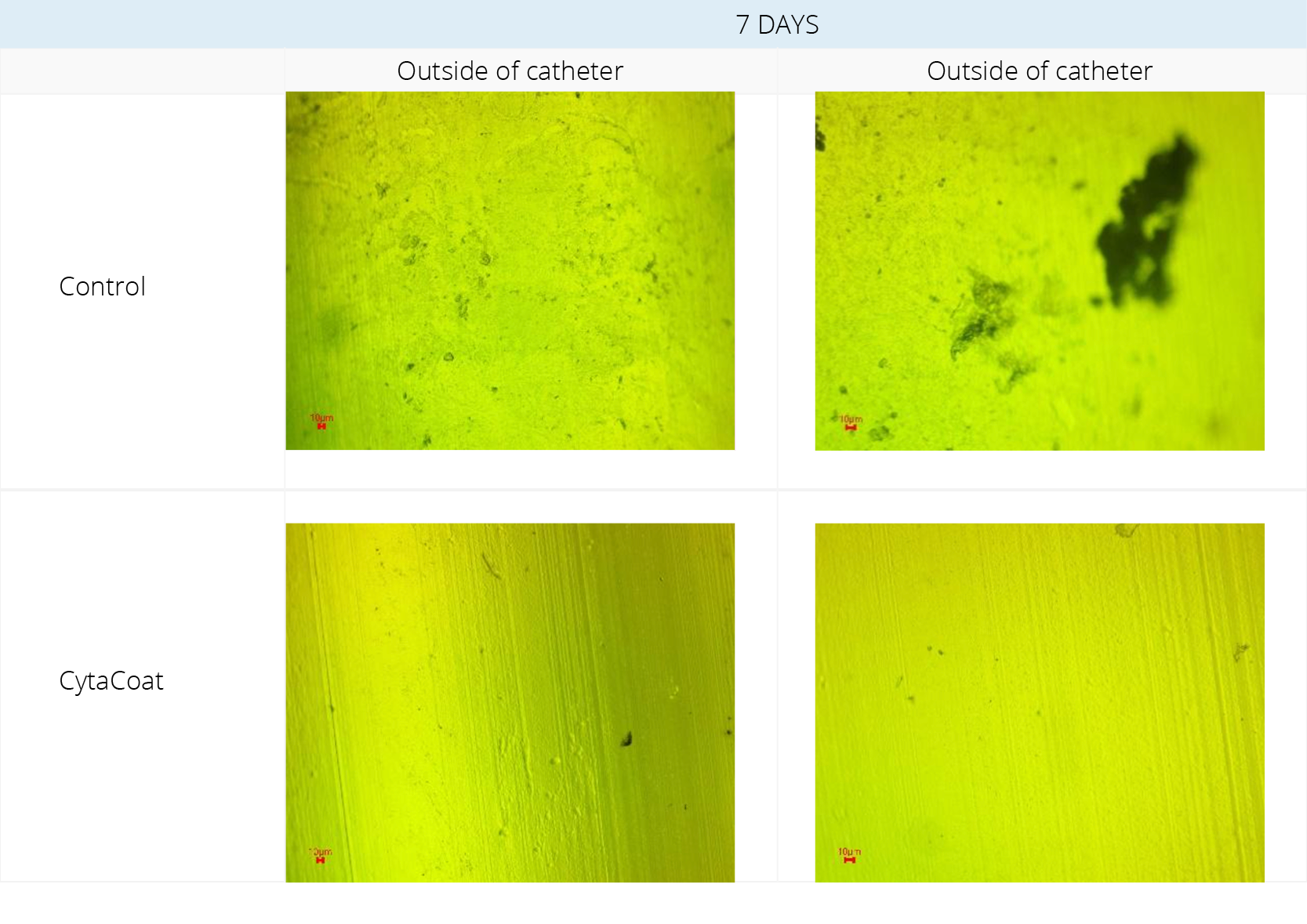
Figure 1. Fluorescent Microscopy visualization before staining.
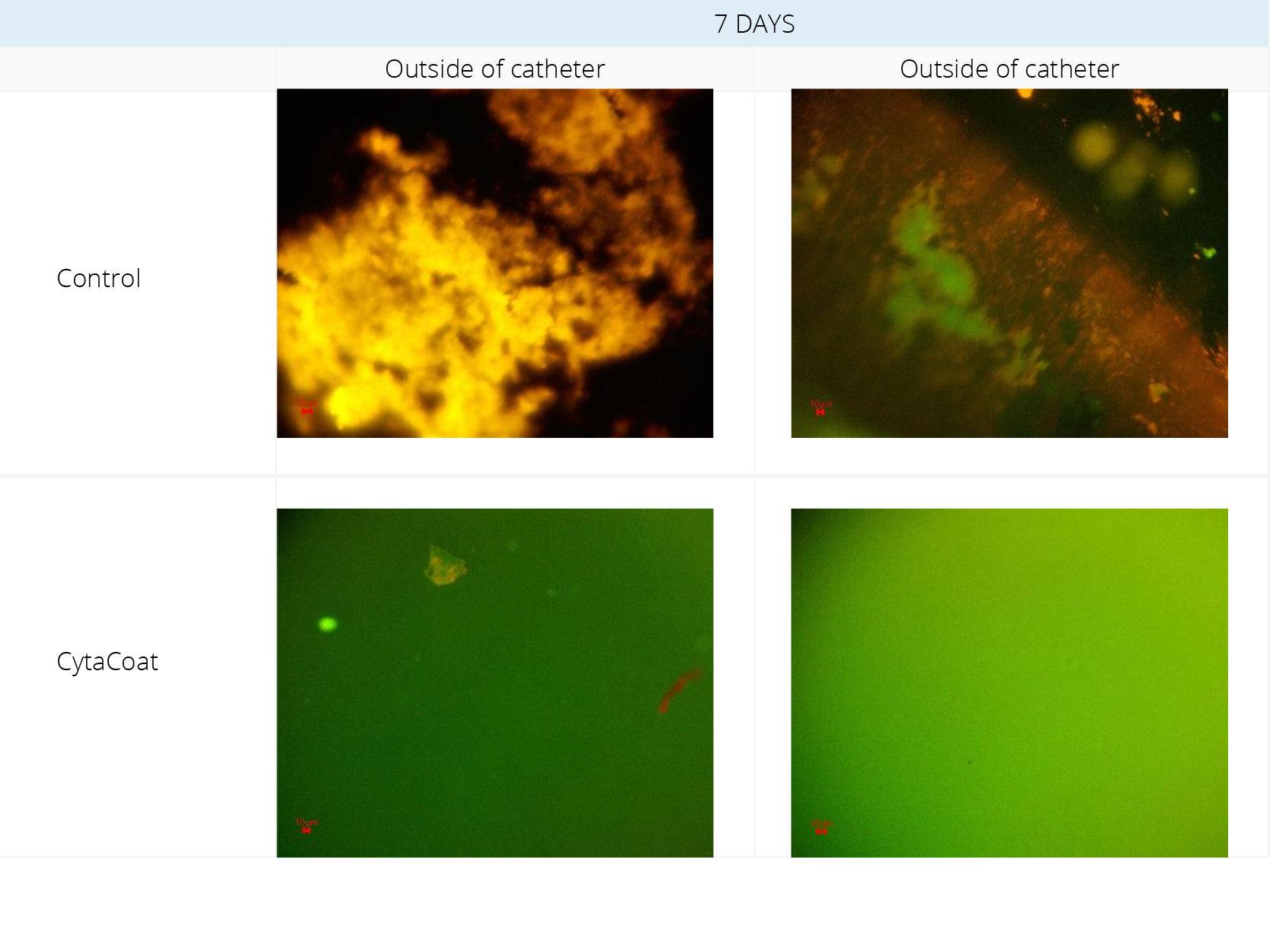
Figure 2. Fluorescent Microscopy visualization after staining
Example: CytaCoat LIP Foley catheter exposed to Klebsiella pneumoniae
Klebsiella pneumoniae was incubated for 7 days in Artificial Urine Medium (AUM) with samples of control and CytaCoat LIP Foley catheter.
A high degree of fouling and biofilm (EPS) formation can be observed on the control sample in Figure 1 (black color) whereas the CytaCoat coated sample is clean on both the inside and outside
In Figure 2, a lot of “stained” biofilm (EPS) was observed on the control for both the inside and outside of the catheter. Very small amounts of biofilm on a “struvite” crystal were seen lying on the outside of the CytaCoat LIP Foley catheter but no biofilm (EPS) could be detected directly on the coated surface and nothing on the inside of the catheter. The green color is just light from the fluorescent microscope. Struvite is common in the urine when urease-producing bacteria like Klebsiella pneumoniae is present.
CytaCoat AB, Maria Aspmans gata 44A, 171 64 Solna SWEDEN | E-mail info@cytacoat.se
Design LIVE Reklambyrå
